Metallic Aromaticity: A New Era in the Study of Heavy Element Bonding
A new era in the study of aromaticity has been ushered in by a team of researchers from Germany, who have successfully synthesized a cationic Bi44+ aromatic ring in a laboratory setting. This marks the first time an all-metal ring with aromatic properties has been isolated without the presence of non-metal stabilizing substituents. The unique structure of this bismuth species has raised important questions about the nature of aromaticity in materials composed of heavier elements.
The creation of similar compounds using only metal atoms has long proven to be a significant challenge, despite centuries of study. While students often learn about aromatic carbon rings such as benzene, the distribution of charge and electron density in metal rings can play a crucial role in determining their bonding character. In recent years, spectroscopic evidence of aromatic all-metal species Al42˗ and antiaromatic Al44˗ was reported, but the isolation of aromatic metal rings, such as those composed of gallium, gold, and thorium, has required the presence of non-metal stabilizing substituents.
The cationic Bi44+ ring exhibits isoelectronic properties with the antiaromatic Al44˗ species, suggesting that the distribution of charge in an ionic aromatic metal ring can determine its bonding character. Interestingly, this discovery challenges traditional models of aromaticity based on resonance hybridization between resonance hybrids and localized p orbitals on carbon atoms.
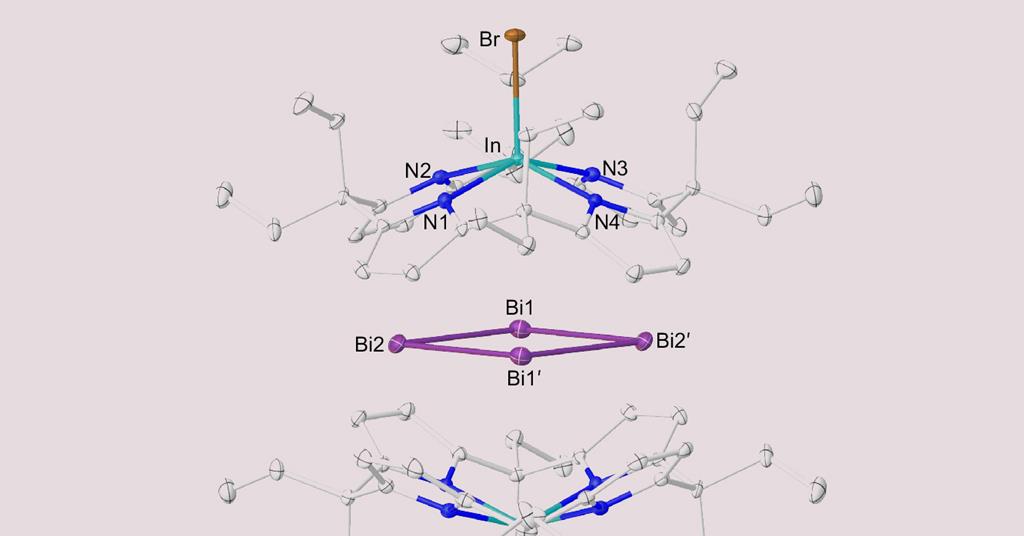
The Bi44+ ring forms a planar rhomboid shape and is held together non-covalently between two shells, each containing an indium bromide core bound within a cyclic ligand composed of four pyrrole units. This unique structure poses challenges for chemists seeking to understand how electrons move within metallic frameworks.
Overall, this groundbreaking discovery sheds light on the complex nature of aromaticity in metal-containing compounds and opens up new avenues for further exploration in the field of inorganic chemistry. It also highlights the need for more research into how different elements interact with one another to create novel materials with unique properties.

